Our Vision and Values
In recent years, COVID-19 has been the most discussed topic and continues to generate a large amount of data. Now, more attention is being given to post COVID-19 condition (PCC) or “long COVID”, long-term effects experienced after a COVID-19 infection.
Policies and practices for managing PCC vary which make evidence-based guidance essential for informing rational decision-making. Research literature may not always provide direct evidence to support or reject policies or practices. However, even knowing the limits of the available evidence can support more informed decision-making and setting research priorities.
To meet this need, the Cochrane Canada and McMaster GRADE Centre at McMaster University are scientifically and financially supported by the Public Health Agency of Canada (PHAC), to provide easily accessible and high-quality guidelines on PCC and facilitate their use in different settings. We will gather input from clinicians, policymakers, equity-seeking populations, and other members of the public. With their feedback, we aim to support the people in Canada with evidence-based recommendations as well as curated tools and resources to improve decision-making.

Our Core Objectives
Create
We will develop six guidelines based on rigorous, clear, and transparent scientific methods that cover the full cycle of post COVID-19 conditions. We will provide you with practical, evidence-based information related to PCC; and facilitate the development of adaptable policies and programs that improve the health of people in Canada.
Disseminate
We will build awareness, share knowledge, inform decision-making, and promote the use of the guidelines through dissemination and implementation activities among clinicians, decision-makers, equity-deserving populations, and the public in Canada. In other words, we want to make these guidelines accessible and understandable for you.
Evaluate
We will evaluate how effective the guidelines are among people who will use the recommendation and conduct quality improvement to optimize the use of PCC guidelines.
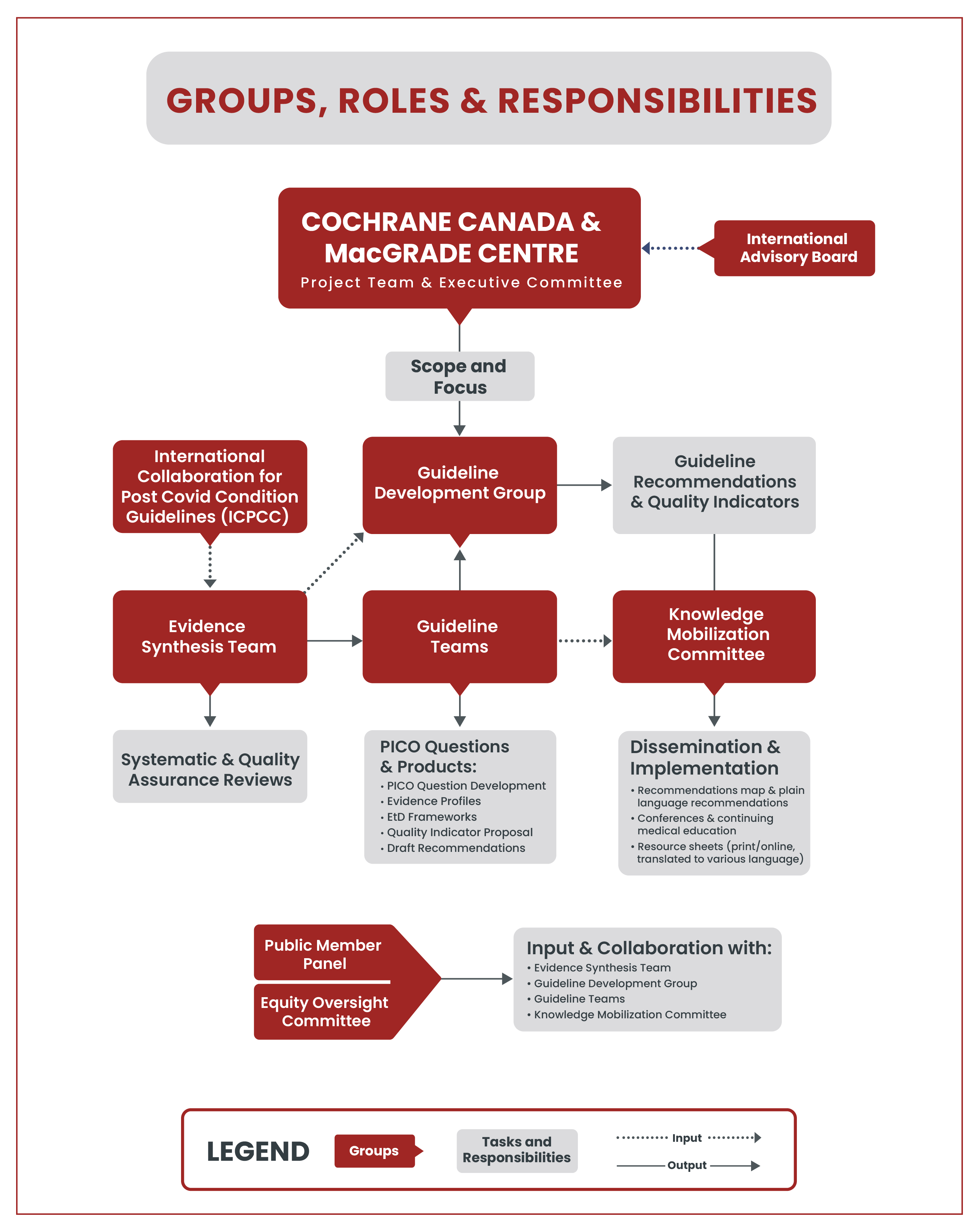
What are the different working groups?

Guideline Teams
Six guideline teams will be formed, each addressing a specific topic area and will include 10-15 representatives with expertise from multiple backgrounds. These teams will be led by topic and methodology experts, will work with the systematic review team to draft recommendations and will then present them to the guideline development group (GDG) for final approval. Guideline team membership will involve discussions in regular online meetings and reviewing documents to complete the following tasks: prioritizing questions and outcomes for guidelines, providing input on evidence and contextual factors, reviewing evidence summaries and profiles, making judgements on evidence and formulating recommendations, reviewing and writing draft recommendations and guideline reports.

Guideline Development Group (GDG)
The GDG is the decision-making body that will make all final decisions on the recommendations. Specifically, the GDG will provide input on the guideline development processes, approve the prioritized topics, questions and outcomes for the guidelines, approve draft recommendations and guideline reports, collaborate in the dissemination and knowledge mobilization of the guidelines, collaborate in the evaluation of the guideline development and dissemination work and will be responsible for creating a plan for updating guidelines.

International Advisory Board (IAB)
The IAB will provide input, expertise and advice on the development process throughout the entire duration of the project. It will guide the executive committee, which is the decision-making body for the guideline project.

Equity Oversight Committee
The equity oversight committee will guide health equity considerations within the project. This committee will play an advisory role in helping to identify priorities for equity considerations, determine methods for and give input on evidence and scoping reviews, and help draft equity considerations for guideline recommendations. This group will include representatives of equity-seeking populations and community leaders to work with equity researchers.

Public Member Panel
The panel will include members of the public and community representatives to adequately reflect the Canadian population and will provide feedback and external input at different stages of the guideline process. The panel will be involved in many tasks, such as prioritizing questions, rating health outcomes, advising on evidence synthesis to consider perspectives of the public and patients, and supporting the knowledge translation of the guidelines.

Evidence Synthesis (ES) Team
For each guideline team and PCC topic, an evidence synthesis team will be tasked with conducting evidence syntheses. Each ES team will have one lead who will be responsible for the specific topic and who will work closely with the guideline team methodologists. The ES teams will be drawn from Cochrane Canada and the McMaster GRADE Centre and international collaborators.

Knowledge Mobilization (KM) Committee
The knowledge mobilization (KM) committee ensures that the six PCC guidelines are accessible, understandable, and are widely used by a range of knowledge users such as clinicians, policymakers, and members of the public. This group is composed of representatives who have KM experience and are from various existing CAN-PCC guideline groups. Examples of KM products will be developed include online resource sheets, interactive infographics, and other online self-management tools.

International Collaboration for Post COVID-19 Guidelines (ICPCC)
The International Collaboration for Post COVID-19 Guidelines (ICPCC) is a voluntary partnership of key global organizations that have related goals of conducting evidence synthesis for the development of recommendations for Post COVID-19 Condition (PCC). The aim of the collaboration is to accomplish evidence identification and synthesis on PCC in an efficient manner, explore ways of agreeing on what evidence might inform a specific guideline question, and find a common evidence interpretation and drawing conclusions related to the questions of interest. The goal is not to issue joint recommendations, but to minimize contradicting interpretation of evidence about PCC which, given the nature of the often very low certainty, would be unavoidable if a partnership and inter-organizational discussions were not held.
Project group organization and overview of responsibilities
The figure below visualizes how the working groups fit into the overall project organization and the main tasks.